Vyntus™ ONE Pulmonary Function System
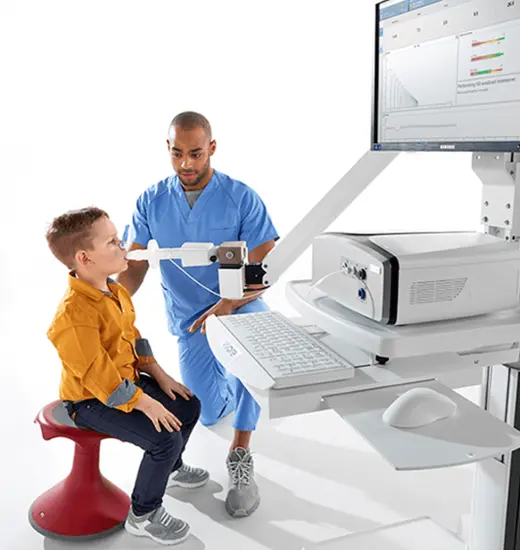
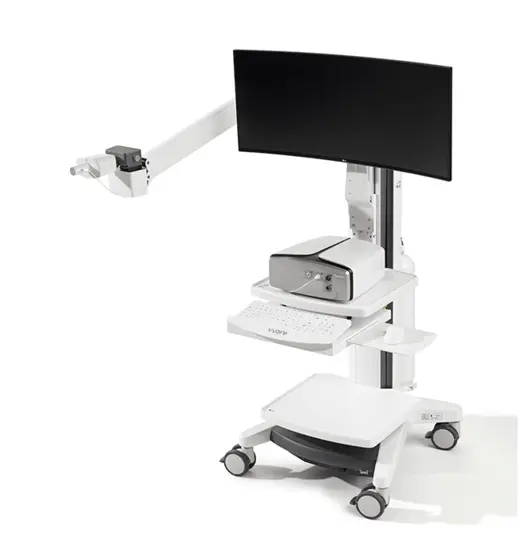






Vyntus™ ONE Pulmonary Function System
The power of ONE - Vyntus™ ONE is our innovative pulmonary function system equipped with an ultrasonic sensor.
Vyntus™ ONE is incredibly capable with a modular design that possesses an impressive array of features. Your Vyntus™ ONE can even grow later on together with your needs delivering high performance and utility in a single package. It is even able to be extended to also measure cardiopulmonary exercise tests with the same module.
It is designed to make your tests more accurate, less intrusive and less stressful for patients.
Visit our Knowledge Base and learn how the latest SentrySuite software helps you achieve the best possible results according to the latest ATS/ERS 2019 Spirometry guidelines.
Please note, all products, services, or features of products and services may not be available in your local area. Please check with your local Vyaire representative. The information provided in this site is intended for healthcare professionals.

Breathing circuit innovations are driving the patient comfort:
- The low resistance of the ultrasonic sensor allows comfortable breathing through the device.
- Due to the innovative design of the flow path valve testing is easier and noise reduced and also results in more successful tests the first time.
- The eDemand valve improves the patient experience during gas inhalation, makes measurements less demanding and also increases the number of successful tests.
The hygienic focus combined with our MicroGard™ filters stops cross-contamination.

- Two sensors - two hygienic concepts avoiding conflicts. Pulmonary function tests are not influenced by cardiopulmonary exercise tests and vice versa. While the digital volume transducer needs to be cleaned after each measurement (no MicroGard filter possible), the ultrasonic sensor only needs to be cleaned every three months.
- Waterproof ultrasonic sensor. There is no need to dis- and reassemble the sensor for the cleaning process.
- Six months cleaning cycle of downstreamed parts using the MicroGard filter. Based on the Bio Burden DIN EN ISO 11737-1: Report 18AA0193
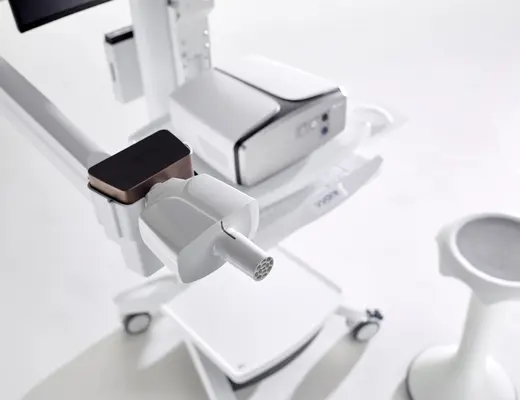
Driving patient centricity
- Ultrasonic sensor with double shot technology, dynamic flow correction and polytubes leading to enhanced data resolution and precision and higher accuracy as well as minimized drifts.
- Innovative flow path valve: Simple, maintenance free, magnetically-controlled rotary shutter is highly responsive to patient effort. This means an easier and noise reduced testing experience as well as testing it right the first time.
- Due to the use of an eDemand valve, the patient no longer needs to maintain a significant under-pressure during gas supply to keep the valve open. This improves the patient experience during gas inhalation, makes the measurement less demanding and increases the number of successful tests.
- Benefit from both trusted and innovative measurement methods. Your Vyntus™ ONE is able to perform Spirometry, Diffusion SB Realtime and Intrabreath as well as FRC N2 Washout measurements. Enhance your PFT lab with an optional bunch of measurements for the lung mechanics.
FRC N2 Washout allows the determination of the functional residual capacity (FRC) using the N2 Washout method as well as the determination of the absolute volumes TLC and RV by combining it with slow spirometry measurements.
- Minimizes your disposables spend as no rebreathing bag, CO2 absorber or tubes are needed.
- Automatic leak detection.
- Workflow-driven: No preparation time before starting the test and automatic switches to the next sequence during the measurement.
- Differentiation of fast and slow lung compartments.
- Measurement of Lung Clearance Index (LCI) to diagnose patients with small airway diseases.


